The draft still needs to be consulted on by groups like the World Trade Organisation (WTO), with the European Coffee Federation (ECF) telling us this meant the claims may not become law until year’s end.
“Even though the EU member states have agreed, and the expert committee agreed to approve the claims, there is further debate to come,” said ECF director Tijmen de Vries.
In cases like this the WTO is typically notified to ensure the regulation changes will not act as a barrier to trade.
Paying attention?
He said the two claims of greatest relevance to the coffee sector – alertness and concentration – may end up being used as background information to support products rather than appearing on-product because of the near ubiquitous public knowledge that caffeine can sharpen mental acuity.
“I can’t speak for coffee companies but the claims might be used as general information on product websites rather than on coffee products. Coffee has never been marketed this way before so we’ll see.”
ECF was happy with the European Commission draft which “was in line” with last year’s European Food Safety Authority (EFSA) opinion that caffeine consumption posed no health concerns at up to 400 mg per day for most adults.
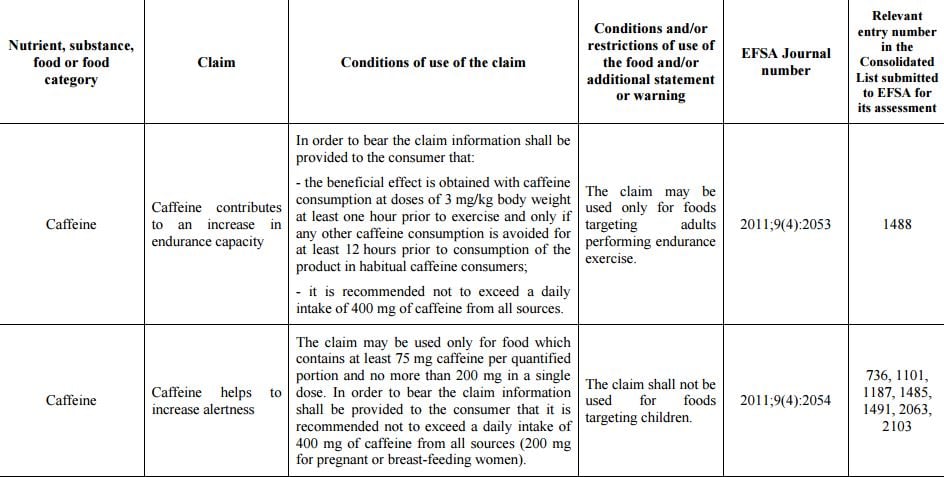
That EFSA opinion recommended lower intakes for groups like pregnant women and said single doses up to 200 mg typically in the form of gels or supplements, were appropriate for those engaged in physical activity.
The four draft claims are applicable when products contain a minimum of 75 mg of caffeine.
Specifically the four claims are:
- Caffeine helps to increase alertness
- Caffeine helps to improve concentration
- Caffeine contributes to an increase in endurance performance
- Caffeine contributes to an increase in endurance capacity
A fifth claim, related to a reduction in perceived effort was not validated because the level of caffeine required to achieve the benefit (4 mg / kg body weight) was deemed to be too high.
Groups like the European Consumer Organisation (BEUC) have warned caffeine claims could promote over-consumption. 400 mg of caffeine equates to about five standard coffee cups or energy drinks.
While EFSA set a safe level of 400 mg per day, agencies in other EU member states like Germany have expressed concerns about over-consumption via formats like energy drinks.
Sleep on it
One part of EFSA's risk assessment was an evaluation of the effects of single and repeated doses of caffeine consumed within a day on the central nervous system of adults (sleep, anxiety, perceived exertion during exercise and subjective perception of alcohol intoxication) and children (sleep, anxiety and behavioural changes).
On this end point it concluded: "The Panel notes that 100 mg of caffeine (about 1.5 mg/kg body weight) may increase sleep latency and reduce sleep duration in some individuals, particularly when consumed close to bedtime."
ESSNA: "very encouraged"

After publication, Dr Adam Carey, chair of the European Specialist Sports Nutrition Association (ESSNA) commented:
“We are very encouraged by and strongly welcome the fact that the claims are recommended for approval, as it will make things a lot clearer for consumers, helping them to find out more information about the products they are purchasing. They key point here is that the Conditions of Use of the two claims aimed at sportspeople are established in relative terms (mg / kg bw) rather than in absolute terms (for instance, 200 mg) – this will allow individual sportsmen and sportswomen, who of course come in many different shapes and sizes, to obtain the claimed benefits regardless of their body shape.
"As these claims pass through the normal decision making process, we very much look forward to them being approved later this year and coming into effect in early 2017.”
